Impact on Industry: Critical Path to TB Drug Regimens
The collaborative work of the CPTR Initiative has contributed to the development of the first new drugs for TB in over 40 years, and has solidified a drug regimen development pipeline in TB.
CPTR: Revitalizing the Pipeline
In the United States, tuberculosis (TB) is easily thought of as a disease of the past. In 2018, the U.S. saw only 9,029 total cases of TB and 542 deaths.1,2 Globally, however, an estimated 10 million people fell ill and 1.4 million died from TB. TB is the world’s leading cause of death from a single infectious agent.3 While there are encouraging trends in the global incidence and death rates (a reduction of 9% and 14%, respectively, over the last five years), TB remains a significant global health problem.In 2010, first-line treatment of TB generally included a multidrug regimen of isoniazid, pyrazinamide, ethambutol and rifampin. Several alternative second line drugs were also in use; however, most were associated with significant adverse events, including permanent loss of hearing. Of the drugs available to treat TB, most were older and had been in use Kate O’Brien, 41, is a TB survivor and advocate from the New York City area with a career in TV production. Kate was hospitalized and put into isolation for more than three months with an active TB infection in 2015, while five months pregnant. During her ordeal, Kate was astonished to learn how many people die of tuberculosis each year worldwide, and how little attention the number one infectious disease killer globally receives, especially in the U.S. Angry at the lack of resources and awareness for the disease, Kate is now working with the National Tuberculosis Controllers Association providing community engagement & patient support through We Are TB, a TB Survivor advocacy organization. Adamant about leaving behind a TB-free world for her children, Kate was named a “CDC TB Elimination Champion” in 2019.
Kate O’Brien, 41, is a TB survivor and advocate from the New York City area with a career in TV production. Kate was hospitalized and put into isolation for more than three months with an active TB infection in 2015, while five months pregnant. During her ordeal, Kate was astonished to learn how many people die of tuberculosis each year worldwide, and how little attention the number one infectious disease killer globally receives, especially in the U.S.
Angry at the lack of resources and awareness for the disease, Kate is now working with the National Tuberculosis Controllers Association providing community engagement & patient support through We Are TB, a TB Survivor advocacy organization. Adamant about leaving behind a TB-free world for her children, Kate was named a “CDC TB Elimination Champion” in 2019. “I had life and death growing in me at the same time – and life won,” she’s said of her diagnosis.
“I had life and death growing in me at the same time – and life won,” she’s said of her diagnosis. Kate and Jimmy O’Brien for many decades. For example, rifampin, the most recently approved at that time by the U.S. Food and Drug Administration (FDA) for use in TB, was approved in 1971.4 Despite the World Health Organization declaring TB a global public health emergency in 1993, it had been more than 40 years since any new drugs were available to treat TB.
At the same time, drug resistant, multidrug resistant (MDR), and extensive drug resistant (XDR) strains of TB were becoming increasing problems. Factors leading to drug resistance are complex and multifactorial. Treatment of TB is lengthy, often required to be at least six months in duration. The treatment course can be complicated in nature, generally consisting of a four-drug regimen for two months, followed by a longer phase of two drugs. The TB mycobacteria itself can lay dormant in the lungs, regrowing and causing reinfection despite lengthy treatment. Populations likely to be infected are from poor and rural countries with limited access to healthcare.5 These factors, and others, all contribute to the emergence of drug-resistant strains of TB that make treating infections even harder.
An urgent need existed for novel drug regimens with improved efficacy and safety profiles, as well as shorter treatment duration, to address the global health crisis in tuberculosis.
To help meet this need, the Critical Path to Tuberculosis Drug Regimens (CPTR) Initiative was launched in March 2010 by Critical Path Institute (C-Path), Bill & Melinda Gates Foundation and Global Alliance for TB Drug Development. CPTR is a public-private partnership that includes the pharmaceutical industry, academic researchers, patient advocates, and national and global regulatory and public health agencies. CPTR has worked over the last 10 years to reinvigorate the drug regimen development pipeline in TB at a time when many pharmaceutical companies were leaving the anti-infective space.
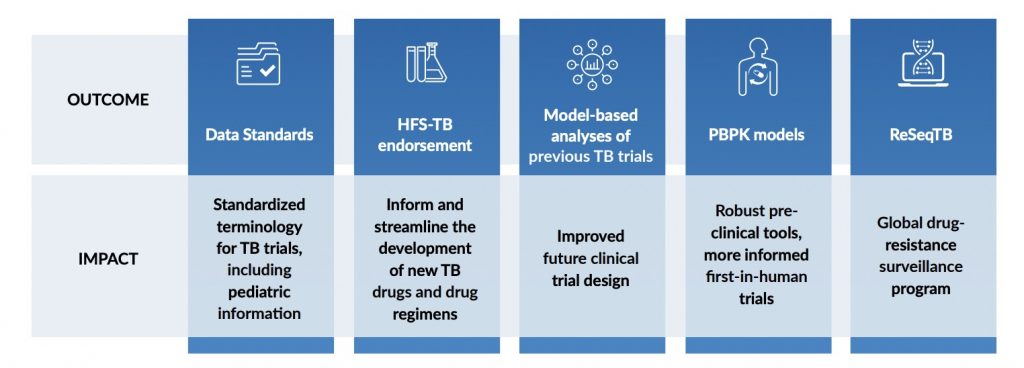
 The results and impact from CPTR’s work have been broad and far-reaching. In partnership with the Clinical Data Interchange Standards Consortium (CDISC), CPTR has developed standardized terminology to support TB clinical trials, including pediatric information.6 CPTR has also received regulatory endorsement of the hollow fiber system of TB, a preclinical model to evaluate dosing requirements for TB drugs used individually or in combination.7 TB-ReFLECT, a model-based analyses of several previously completed TB trials, has enabled learnings that will help the community understand study outcomes and improve the design of future clinical trials.8 In partnership with Simcyp™ scientists, CPTR has developed a TB-specific set of physiologically-based pharmacokinetic models and compound files that will inform first-in-human clinical studies.9 CPTR has also partnered with the Translational Genomics Research Institute’s (T-Gen’s) Pathogen Genomics Division to whole genome sequence TB isolates with clinical and culture-based drug sensitivity data.10 This data was incorporated with similar data from the global community and able to identify drug resistance patterns across the globe. Incredibly, this is an incomplete list of CPTR’s successes to date.
The results and impact from CPTR’s work have been broad and far-reaching. In partnership with the Clinical Data Interchange Standards Consortium (CDISC), CPTR has developed standardized terminology to support TB clinical trials, including pediatric information.6 CPTR has also received regulatory endorsement of the hollow fiber system of TB, a preclinical model to evaluate dosing requirements for TB drugs used individually or in combination.7 TB-ReFLECT, a model-based analyses of several previously completed TB trials, has enabled learnings that will help the community understand study outcomes and improve the design of future clinical trials.8 In partnership with Simcyp™ scientists, CPTR has developed a TB-specific set of physiologically-based pharmacokinetic models and compound files that will inform first-in-human clinical studies.9 CPTR has also partnered with the Translational Genomics Research Institute’s (T-Gen’s) Pathogen Genomics Division to whole genome sequence TB isolates with clinical and culture-based drug sensitivity data.10 This data was incorporated with similar data from the global community and able to identify drug resistance patterns across the globe. Incredibly, this is an incomplete list of CPTR’s successes to date.
In 2012, bedaquiline was approved by FDA for use as part of combination therapy for MDR TB.11 In 2019, pretomanid received FDA approval for use as part of a combination regimen with bedaquiline and linezolid for the treatment of XDR and MDR TB.12 After more than 40 years of stagnation, patients around the globe have the first new drug regimens for TB. The years of collaborative work of the CPTR initiative has contributed to these approvals, and most importantly, helped solidify a drug regimen development pipeline to meet the needs of TB patients everywhere.
Critical Path Institute is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) and is 55% funded by FDA/HHS, totaling $14,575,306, and 45% funded by non-government source(s), totaling $11,916,747. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, FDA/HHS or the U.S. Government. For more information, please visit FDA.gov.




