Impact For Patients: Transplant Therapeutics Consortium
TTC’s work to qualify a reasonably likely surrogate endpoint will speed up drug development for kidney transplant patients.
Haley Jensen enjoys being active and relishes the outdoors, side effects she attributes to growing up in Evergreen, Colorado, a small town of about 10,000 people 30 miles west of Denver. The eldest of four siblings, Haley remembers her early childhood as that of any other kid from Evergreen: filled with soccer, hiking, skiing, swim team, and anything else that kept her and her siblings active.
But in high school Haley began to feel constantly fatigued. She had unexplained nose bleeds and other symptoms that drifted further and further away from that normal Evergreen childhood. Then, over Memorial Day weekend, Haley became sick enough that her dad took her to the local urgent care. The nurses struggled to measure her blood pressure, but when they did, they found it was dangerously high, and she was rushed by ambulance to the emergency room for care. With a sudden unexpectedness she describes as “a bolt of lightning,” Haley’s life changed forever.
At the hospital, Haley’s doctors diagnosed her with idiopathic kidney disease, kidney disease with no known cause, and she was immediately put on dialysis. Haley’s doctors encouraged her to undergo peritoneal dialysis from home, and told her she would need to continue dialysis indefinitely, unless she could receive a kidney transplant.
 For Haley, home dialysis was the best option by far. However, despite avoiding multiple hours-long visits to the dialysis center each week, home dialysis was still made difficult by constant physical, mental, and emotional exhaustion. Haley, with the support of her family, did her best to live a normal life. She continued to go to school and be as active as possible, at times even being wrapped up in hockey pads by her dad to keep her safe while playing soccer. She swam as much as possible, but was limited by feeling chronically unwell and fatigued. Although Haley loved to eat good food, dialysis came with severe dietary restrictions and she found she had little appetite. Worse, Haley had a constant fear of infection and there was an emotional toll from the ever-present machines in the bedroom she shared with her little sister. While at times her days could feel somewhat normal, for six months Haley had to hook up to those machines every evening, cementing the impossibility of a “normal” life.
For Haley, home dialysis was the best option by far. However, despite avoiding multiple hours-long visits to the dialysis center each week, home dialysis was still made difficult by constant physical, mental, and emotional exhaustion. Haley, with the support of her family, did her best to live a normal life. She continued to go to school and be as active as possible, at times even being wrapped up in hockey pads by her dad to keep her safe while playing soccer. She swam as much as possible, but was limited by feeling chronically unwell and fatigued. Although Haley loved to eat good food, dialysis came with severe dietary restrictions and she found she had little appetite. Worse, Haley had a constant fear of infection and there was an emotional toll from the ever-present machines in the bedroom she shared with her little sister. While at times her days could feel somewhat normal, for six months Haley had to hook up to those machines every evening, cementing the impossibility of a “normal” life.
When the call finally came that a kidney was available, she was so excited to say goodbye to her machines she didn’t have time to worry about the massive surgery she was about to undergo. Thanks to Haley’s amazing team at Children’s Hospital Colorado, the benefits of the procedure were immediate. Haley recalls her parents saying she “looked better coming out of surgery than she had going in.” After surgery, the first thing Haley did was have as much chocolate and milk as she could, something her diet didn’t allow during her months on dialysis.
Haley says she quickly felt better after her procedure, but admits there were major adjustments. Although Haley says she “will do whatever it takes to keep her organ healthy” to avoid needing dialysis again, she has found that keeping her kidney healthy is a full-time job. In addition to needing regular specialty care from a team of clinicians, Haley must eat well, exercise regularly, stay hydrated, and live as healthy a lifestyle as possible. Most importantly, Haley says her days often seem to revolve around the regimen of at least seven different medications she takes multiple times each day as a result of her transplant. Haley takes so many medications she has had to adopt an abundance of techniques to make sure she always has medication available and remains adherent to her regimen.
Many of her medications prevent her immune system from rejecting her kidney, referred to as “immunosuppressive therapies,” but many others are needed to treat the physical and emotional side effects that these life-saving therapies cause. Haley has experienced tremors, memory problems, dietary issues and many other side effects. Haley says she sometimes struggles to know if a new symptom is a side effect of her drugs or something new. Haley also carries the emotional burden of worrying about the long-term risk of life-threatening infections, skin and other rare cancers and the difficulties she will likely face having a family with her new husband.
“This matters. Part of the reason why I have a kidney is to be able to live my life, and I want to be able to live it.”
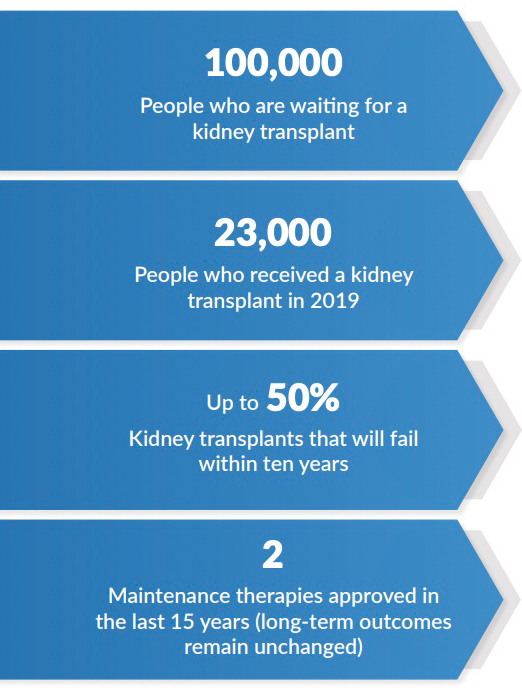
Haley knows that even under the best circumstances, complications around her transplant will inevitably arise. “Eventually, something is going to go wrong, and something is going to happen to my transplant,” Haley says. While these medications are “tremendously appreciated” and Haley “wouldn’t trade them for the world,” the currently available medications have significant limitations. Better options for patients like Haley are needed. In addition to bad side effect profiles, long-term outcomes for transplant recipients who take the currently available regimens remain unacceptably poor. According to the United States Renal Disease System’s (USRDS) 2019 report, graft-survival rates were 93-98% one-year after transplant, but only 34-50% by 10-years.
Despite the high burden of unmet needs felt by kidney transplant recipients and poor long-term outcomes, drug development for this condition has remained largely stagnant for decades. The most widely cited barrier to development is the lack of “surrogate endpoints” that would allow shorter clinical trials to predict long-term outcomes. Without this type of endpoint, clinical trials must be large and/or lengthy, and therefore expensive, in order to determine the long-term benefits of a new therapy. In the face of high costs of development, drug companies have turned their attention to other conditions, leaving patient needs unmet.
To meet this need and to help provide access to better medications for kidney transplant recipients, the Transplant Therapeutics Consortium (TTC) was co-founded in 2017 by the Critical Path Institute (C-Path), the American Society for Transplantation and the American Society for Transplant Surgeons. TTC is a public-private partnership of the transplant community, and includes the pharmaceutical industry, clinicians, academic researchers and regulatory agencies. TTC’s mission is to accelerate the pace of medical product development for transplant recipients, focusing first on kidney transplantation.
Building on over a decade of work by Dr. Alexandre Loupy and the Paris Transplant Group, TTC aims to decrease the time required to bring a new product to patients by seeking regulatory endorsement of the iBox Scoring System as a surrogate endpoint capable of predicting long-term kidney transplant outcomes.
 To achieve this goal, TTC is pursuing qualification of the iBox Scoring System through the Food and Drug Administration’s (FDA) Biomarker Qualification Program and the European Medicines Agency’s (EMA) Qualification of Novel Methodologies pathway. Qualification will require a large amount of data from a wide variety of sources. TTC leverages the pre-competitive space it creates to carry out a global data sharing effort, which now includes data from 11 clinical trial or clinical center sources, representing over 15,000 kidney transplant recipients.
To achieve this goal, TTC is pursuing qualification of the iBox Scoring System through the Food and Drug Administration’s (FDA) Biomarker Qualification Program and the European Medicines Agency’s (EMA) Qualification of Novel Methodologies pathway. Qualification will require a large amount of data from a wide variety of sources. TTC leverages the pre-competitive space it creates to carry out a global data sharing effort, which now includes data from 11 clinical trial or clinical center sources, representing over 15,000 kidney transplant recipients.
The iBox Scoring System was recently accepted into FDA’s Biomarker Qualification Program, bringing this tool one step closer to being publicly available for use. While formal qualification of the iBox is likely two to three years away, the transplant community’s knowledge of the data supporting this tool is increasing with each monthly consortium meeting and each new shared dataset, increasing the community’s confidence in the use of this tool.
The potential availability of a new surrogate endpoint has helped to reshape the drug development landscape for transplantation. For the first time in decades, multiple companies are considering clinical trials that will utilize this new surrogate endpoint, bringing hope for better treatments to kidney transplant recipients.
Haley, who was recently awarded an MBA and MPH from Boston University, says the most important message she’d like the drug development community to hear is simply, “This matters. Living with a kidney transplant is not easy, but is infinitely better than not having one at all,” Haley says. “One of the many great things about having a transplant is, despite the difficulties, it’s not enough to keep me from living my life and thinking about the future. Part of the reason why I have a kidney is to be able to live my life, and I want to be able to live it.”
Critical Path Institute is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) and is 55% funded by FDA/HHS, totaling $14,575,306, and 45% funded by non-government source(s), totaling $11,916,747. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, FDA/HHS or the U.S. Government. For more information, please visit FDA.gov.



